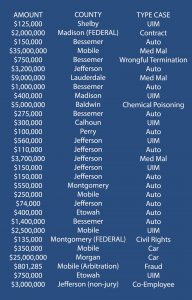
Any attorney representing individuals injured in accidents understands that an injured person, or the plaintiff rather, has the burden of proving the essential elements of their claim. One of the essential elements of any personal injury claim is causation. There are two components to causation. First, the plaintiff is required to establish that the defendant’s conduct caused the event in question and secondly, the plaintiff is required to establish that the subject event caused the claimed injuries. However, what does it mean to establish sufficient proof of “causation” in a personal injury case.
Almost every attorney assessing injury causation will approach the analysis from the standpoint that testimony is needed by a qualified healthcare provider that the injuries were likely caused, or probably caused, by the underlying event. Attorneys often use a phrase “within a reasonable degree of medical probability or certainty” to describe the injury causation standard. Another way to view this standard or burden of proof is to view “probability” as meaning greater than 50%.
 Alabama Injury Law Blog
Alabama Injury Law Blog
















 by HWC Managing Partner Josh Wright
by HWC Managing Partner Josh Wright